Life Sciences research, improve the innovation, able to influence clinical decision making, both in diagnosis and therapy. Indeed, from the knowledge of the special needs and demands of the technical-scientific, researchers were able to contain and experiment innovative solutions. Despite these strengths, transferring research to the market in the Life Sciences shows criticalities enough.
The purpose of this paper is to provide concrete evidence of the technology transfer process based on the exploitation of the results obtained by KronosDNAsrl, an academic spin-off focusing on reproductive medicine.
method
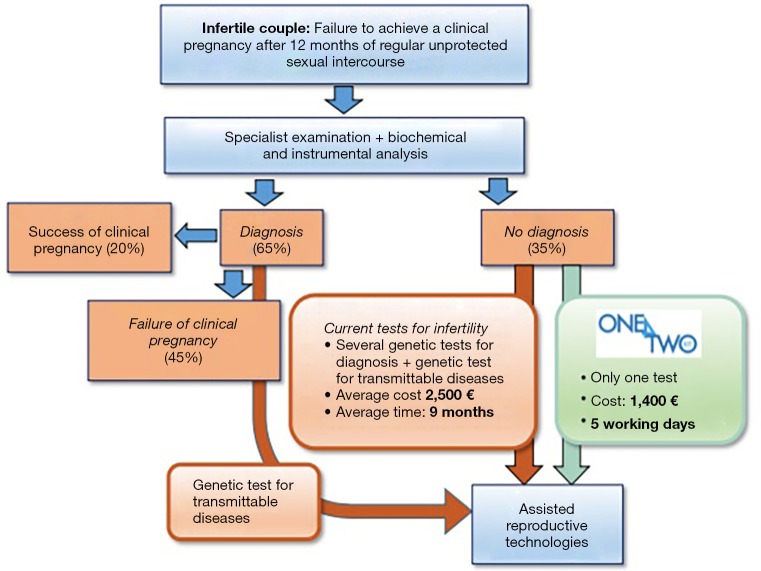
Different tools are used to evaluate the technical feasibility (validation of the results obtained with the prototype) and to manage the process of technology transfer from One4Two®.
result
Different analyzes we conducted demonstrated the feasibility of the proposed solution. As a result, a prototype has been developed and validated One4Two®.
conclusion
Here, we provide evidence of how the power of knowledge acquired translational research on the “bench” can be used to transfer to the market on a “benchmark” allows innovation in Laboratory Medicine. In addition, the model described for One4Two® can be easily transferred to other products.
Scientific integrity among nursing students participating in the Scientific Initiation Program: An exploratory study.
To determine the position and practice adopted by nursing students in scientific initiation program on the principles of scientific integrity in various stages of the process of doing science.An quantitative exploratory study, in which participants from the nursing students Scientific Initiation Program of the federal District interviewed.
Fifty (50) nursing students participated in the study. Most participants were interviewed presented a good understanding of the process of doing research in different stages. However, it was found that although they were familiar with the practices of good science, students do not always behave in the most responsible way.
It was observed that the knowledge on topics related to the ethics of the scientific process is mainly obtained through formal education, which consists of classes and courses. Nevertheless, the importance of complementary spaces such as study and research groups recognized.
Conclusion: Research experience that education and vocational training room are important for students. Hence, good research practice need to be incorporated at the beginning of the academic curriculum.